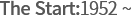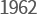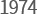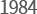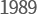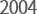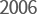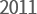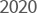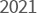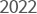-

- Importing medicine
from JP & US
-

- Establishment of
Yungjin Pharm
-

- First API plant introduce Penicillin in domestic market
-
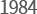

- The world's first Bacampicillin synthesis
-
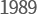

- Factory for Finished products in Namyang
-
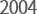

- Joined as a member
of KT&G Group
-
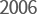

- New Jeonju Factory for API production
-

- New Cephalosporin plant in Namyang factory
-

- '$70M Export Prize'
awarded by KITA
-
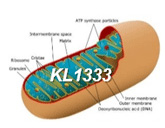
- KL1333 WW rights licensed to Abliva(excl. KR, JP)
-
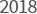

- Received ISO37001
certification
-
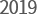

- YPN-005 oral
presentation at AACR
-
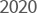

- Received Family-friendly
company certification
from The Ministry of
Gender Equality and
Family
-
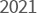

- The completion of
YUNGJIN BIO INDUSTRIAL
PARK in Hwaseong
-

- Received ISO14001/45001
certification